
"We can help researchers from any field who require light microscopy"
The facility, based at Queen Mary University of London's Charterhouse Square campus, has grown from expertise and infrastructure acquired by the CMR research groups to become an open-access facility with a dedicated facility manager.
Equipment
Inverted confocal laser scanning microscopy




Zeiss LSM800 microscope
Our Zeiss LSM800 is a fully motorised inverted microscope with a fast piezo Z-drive ideally suited to live cell imaging, high-resolution fixed sample analyses or capturing detailed tile-scans of tissue sections.
Full CO2 & temperature control alongside the Zeiss Definite Focus system can accommodate long-term time-lapse experiments. In addition to the basics, you can create complex acquisitions with the flexible Zeiss 'experiment designer', tile and stitch to capture large tissues at high-resolution, carry out FRAP experiments or multi-well plate scans.
405, 488, 561 and 640 nm solid state laser diodes
2 GaAsP PMT detectors, 1 transmitted light detector
10x/0.3 Neofluar, 20x/0.8 Plan Apochromat, 40x/1.3 oil Plan Apochromat, 63x/1.4 oil Plan apochromat
Upright confocal laser scanning microscopy




Leica SP5 and Leica SP8 microscopes
Our two Leica upright confocal laser scanning microscopes are equipped with a large stage area, water dipping lenses and optional high-speed resonant scanner to enable live tissue imaging applications in addition to standard fixed sample imaging.
SP5: Argon laser (458, 476, 488, 496, 514 nm lines), DPSS laser (561 nm) and HeNe laser (633 nm) ; SP8: Tunable pulsed 1.5 mW white light laser, up to eight simultaneous lines between 470-670 nm.
SP5: 3x PMT ; SP8: 2 PMT and 2 Hybrid detectors
SP5: 20x/1.0 W Apo (2.0 mm WD) as standard ; SP8: 20x/1.0 W Apo (2.0 mm WD) as standard.
Multiphoton & Second Harmonic Generation imaging




Leica SP8 DIVE microscope
Multiphoton microscopy enables deeper imaging than confocal techniques and reduced photobleaching by selectively exciting the focal plane alone.
Our state-of-the-art Leica SP8 DIVE microscope can function as a confocal or multiphoton. For the latter it includes a tunable 680-1300 nm IR laser plus a fixed 1045 nm laser and four detectors (two highly sensitive hybrid detectors and two PMTs). Our long working-distance objective lenses allow a deep imaging range and the system is built to handle live tissue imaging too.
488, 552, 638 nm for confocal; InSight DS+ DUAL tunable 680-1300 nm IR pulsed solid state laser, plus 1045 nm fixed line for multiphoton.
2 confocal PMTs, 4 non-descanned detectors for multiphoton imaging (2x PMT and 2x Hybrid detector)
25x/1.0 W IR Apo with motCorr (2.6 mm WD) as standard.
High-resolution Spinning Disk

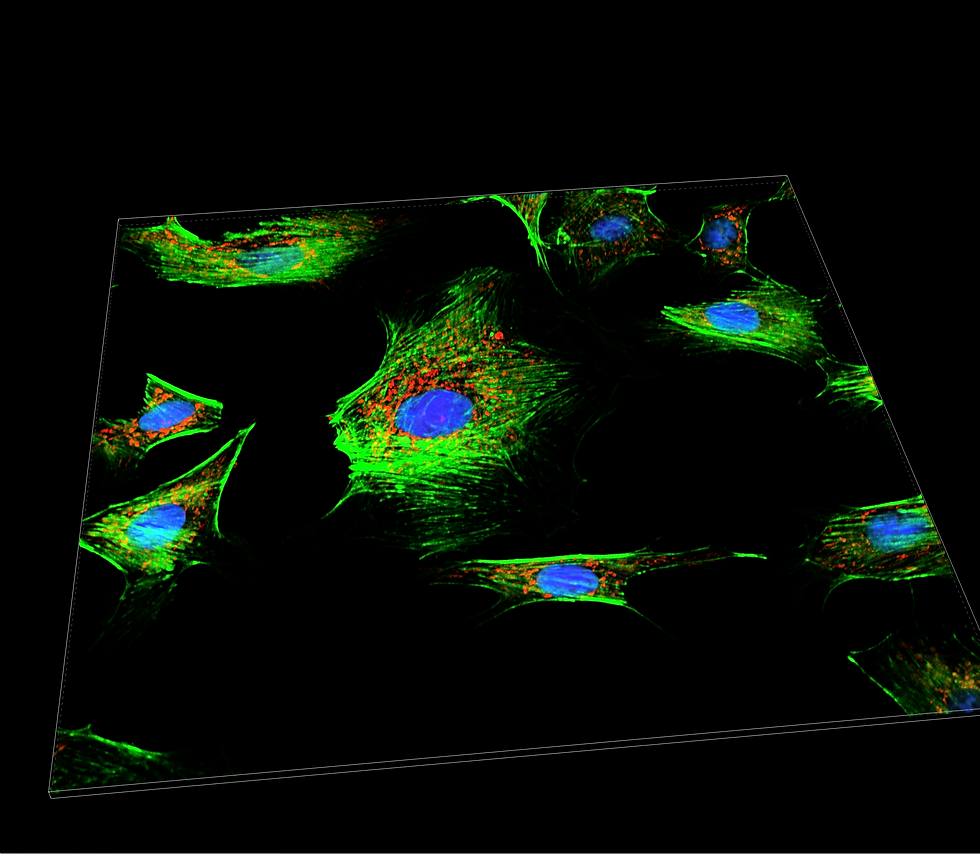


Nikon W1 SoRa
Nikon's Spinning Disk SoRa W1-SoRa combines confocal microscopy with optical photon reassignment (SoRa) for dual-mode imaging. SoRa's micro-lens pinholes effectively double resolution compared to standard confocal, enabling detailed visualization of cellular structures and interactions. This hybrid system offers both high-resolution confocal imaging and rapid acquisition without complex computational deconvolution, making it valuable for diverse biological research applications.
Finally, the W1-SoRa integrate an incubation chamber with controlled temperature and CO2, which is crucial for accurate and successful live-cell imaging.
Omicron LightHUB 405nm, 488nm, 561nm and 638nm
2x Photometrics BSI Express 4.2 megapixel camera, 95% QE, 6.5um x 6.5um pixel area, FOV 18.8 mm in diameter.
10x, 20x, 40x oil, 40x sil (tissue), 60x oil, 100x oil and 100x sil (tissue)
Fluorescence lifetime imaging microscopy (FLIM) & FRET applications



Leica SP8 FALCON microscope
Fluorescence lifetime imaging microscopy (FLIM) captures the decay time of the fluorophore while building a traditional fluorescence image. The lifetime of a fluorophore is sensitive to its local environment and can be used to detect chemical changes or even FRET between interacting proteins.
Our Leica SP8 DIVE is equipped with a state-of-the-art Falcon module for fast fluorescence lifetime imaging and intuitive image processing.
N/A
N/A
N/A
Image Processing Platform


Imaris, FIJI, Huygens, NIS and ilastik
Four workstations are dedicated to image processing and analyses, with the most advance analyse software such as Imaris for 3D analyses, Huygens for cutting-edge deconvolution processing and many other dedicate to routine analyses, deconvolution and AI machine learning segmentation.
N/A
N/A
N/A
People & expertise

Dr Paul Imbert
Microscopy Facility Manager
Meet Paul, our microscopy expert at the CMR. Paul brings a wealth of experience in confocal microscopy, live cell imaging, large image acquisition, multiphoton techniques and deconvolution. His multidisciplinary background positions him perfectly to provide comprehensive support for your research projects involving microscopy. From hands-on experience in image acquisition to meticulous data analysis and the crafting of grant proposals, Paul is here to elevate your scientific projects.

Dr Tom Nightingale
Advisory Committee
Lecturer in cell biology & research group leader in the field of endothelial cell biology. Nightingale's group employ a range of techniques to understand the intracellular trafficking of proteins regulating key endotheiial functions within inflammation and blood clotting. In studying these dynamic intracellular mechanisms, his group push the limits of resolution and live cell imaging capability.

Prof. Sussan Nourshargh
Academic Lead
Head of the Centre for Microvascular Research & leads internationally rated research in the field leukocyte migration. Nourshargh's team has widely acknowledged expertise in optical microscopy, in particular confocal intravital imaging, having developed novel imaging strategies to track leukocyte behaviour in situ.

Dr Mathieu-Benoit Voisin
Advisory Committee
Leader & expert in intravital microscopy techniques. He employs live tissue confocal and multiphoton approaches, among others, to understand the mechanisms of neutrophil interactions with lymphatic vessels, their migration into lymphoid organs and their role in the regulation of acquired immunity in acute and chronic pathologies.
Stat on the facility 2022
Coming soon.
Users
Groups
Institutes